Chromatography
HPLC Phases
ASTRA - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| C18-HE | 2, 3, 5, 10 | 100 | 330 | 17 | 2-9 |
| C18-AQ | 2. 3, 5 | 100 | 330 | 13 | 2-9 |
| C18-BDS | 3, 5 | 140 | 170 | 11 | 2-8 |
| C8-HE | 5 | 100 | 330 | 11 | 2-9 |
| C8-BDS | 3, 5 | 140 | 170 | 6 | 2-8 |
| Phenyl-Hexyl-HE | 3, 5 | 100 | 330 | 11 | 2-7.5 |
| DM | 3, 5 | 100 | 205 | 12 | 2-9 |
| Diol | 3, 5 | 100 | 330 | 5 | 2-7.5 |
ARION - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| Plus C18 | 1.7, 2.2, 3, 5, 10, 15 | 100 | 420 | 18 | 1.5-10 |
| Polar C18 | 2.2, 3, 5, 10, 15 | 120 | 325 | 16 | 1.5-7.0 |
| C8 | 3, 5 | 120 | 325 | 11 | 2.0-7.0 |
| Phenyl-butyl | 2.2, 3, 5 | 100 | 300 | 12 | 1.5-7.5 |
| NH2 | 2.2, 3, 5 | 120 | 325 | 5 | 2.0-6.5 |
| CN | 3, 5, 10 | 120 | 325 | 8 | 2.0-7.0 |
| HILIC Plus | 2.2, 3, 5 | 120 | 420 | - | 1.5-7.0 |
| Si | 2.2, 3, 5, 10 | 100 | 420 | - | 1.5-7.0 |
| SAX | 5 | 120 | 325 | - | 1.0-7.5 |
| SCX | 5 | 120 | 325 | - | 1.0-7.5 |
More information is available in Column care guide aswell.
CHROMSHELL - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| CHROMSHELL® C18 Plus | 2.6 | 85 | 130 | 9 | 1.5-7.5 |
| CHROMSHELL® C18-XB | 2.6 | 85 | 130 | 8 | 1.5-8.0 |
| CHROMSHELL® C18 Polar | 2.6 | 85 | 130 | 6.5 | 1.5-7.0 |
KINETEX - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Effective Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| Kinetex XB-C18 | 5, 2.6 | 100 | 200 | 10 | 1.5-8.5* |
| Kinetex C18 | 5, 2.6 | 100 | 200 | 12 | 1.5-8.5* |
| Kinetex C8 | 2.6 | 100 | 200 | 8 | 1.5-8.5* |
| Kinetex PFP | 5, 2.6 | 100 | 200 | 9 | 1.5-8.5* |
| Kinetex HILIC | 2.6 | 100 | 200 | 0 | 2.0-7.5 |
| Kinetex Phenyl-Hexyl | 5, 2.6 | 100 | 200 | 11 | 1.5-8.5* |
* Columns are pH stable from 1.5 to 10 under isocratic conditions. Columns are pH stable from 1.5 to 8.5 under gradient conditions.
Kinetex 2.6µm columns with ID 2.1mm are pressure stable up to 1000 bar, otherwise up to 600 bar.
Kinetec chore-shell colums can be replace by new ChromShell colums. Just try it.
LUNA - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Luna Phenyl-Hexyl | 3,5,10,15 | 100 | 400 | 17.5 | 1.5-10.0 | L11 |
| Luna Silica (2) | 3,5,10,15 | 100 | 400 | - | - | L3 |
| Luna C5 | 5,10 | 100 | 440 | 12.5 | 1.5-10.0 | - |
| Luna C8 | 5,10 | 100 | 440 | 14.75 | 1.5-10.0 | L7 |
| Luna C8 (2) | 3,5,10,15 | 100 | 400 | 13.5 | 1.5-10.0 | L7 |
| Luna C18 | 5,10 | 100 | 440 | 19 | 1.5-10.0 | L1 |
| Luna C18 (2) | 2.5,3,5,10,15 | 100 | 400 | 17.5 | 1.5-10.0 | L1 |
| Luna CN | 3,5,10 | 100 | 400 | 7.0 | 1.5-10.0 | L10 |
| Luna NH2 | 3,5,10 | 100 | 400 | 9.5 | 1.5-11.0 | L8 |
| Luna SCX | 5,10 | 100 | 400 | 0.55% Sulfur Load | 2.0-7.0 | L9 |
| Luna HILIC | 3,5 | 200 | 200 | - | 1.5-8.0 | - |
| Luna PFP(2) | 3 5 | 100 | 400 | 5.7 | 1.5-8.0 | L43 |
GEMINI - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Gemini C18 | 3,5,10 | 110 | 375 | 14 | 1.0-12.0 | L1 |
| Gemini C6-Phenyl | 3,5 | 110 | 375 | 12 | 1.0-12.0 | L11 |
| Gemini NX | 3,5,10 | 110 | 375 | 14 | 1.0-12.0 | L1 |
SYNERGI - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Synergi Max-RP | 2.5 | 100 | 400 | 17 | 1.5-10.0 | - |
| Synergi Hydro-RP | 2.5 | 100 | 400 | 19 | 1.5-7.5 | L1 |
| Synergi Polar-RP | 2.5 | 100 | 440 | 11 | 1.5-7.0 | L11 |
| Synergi Fusion-RP | 2.5 | 100 | 440 | 12 | 1.5-10.0 | L1 |
| Synergi Max-RP | 4,10 | 80 | 475 | 17 | 1.5-10.0 | - |
| Synergi Hydro-RP | 4,10 | 80 | 475 | 19 | 1.5-7.5 | L1 |
| Synergi Polar-RP | 4,10 | 80 | 475 | 11 | 1.5-7.0 | L11 |
| Synergi Fusion-RP | 4,10 | 80 | 475 | 12 | 1.5-10.0 | L1 |
ONYX - PHENOMENEX
| Packing Material | Macropore Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Onyx Silica | 2 | 130 | 300 | 0 | 2.0-7.5 | - |
| Onyx C8 | 2 | 130 | 300 | 11 | 2.0-7.5 | - |
| Onyx C18 | 2 | 130 | 300 | 18 | 2.0-7.5 | - |
JUPITER - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Jupiter C4 | 5,10,15 | 300 | 170 | 5.0 | 1.5-10.0 | L26 |
| Jupiter C5 | 5,10,15 | 300 | 170 | 5.5 | 1.5-10.0 | - |
| Jupiter C18 | 5,10,15 | 300 | 170 | 13.3 | 1.5-10.0 | L1 |
| Jupiter Proteo C12 | 4,10 | 90 | 475 | 15.0 | 1.5-10.0 | - |
GraceSmart - GRACE
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| GraceSmart C18 | 3,5 | 120 | 220 | 10 | 2.0-9.0 | L1 |
Alltech® Prevail - GRACE
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Prevail C18 | 3,5 | 110 | 350 | 17 | L1 | |
| Prevail C18 Select | 3,5 | 110 | 350 | 15 | L1 | |
| Prevail C8 | 3,5 | 110 | 350 | 8 | L7 | |
| Prevail Phenyl | 3,5 | 110 | 350 | 7 | L11 | |
| Prevail Cyano (CN) | 3,5 | 110 | 350 | - | L10 | |
| Prevail Amino (NH2) | 3,5 | 110 | 350 | - | L8 | |
| Prevail Silica | 3,5 | 110 | 350 | - | L3 | |
| Prevail Organic Acid | 3,5 | 110 | 350 | - | - | |
| Carbohydrate ES (polymer) | 5 | - | - | - | - |
Nano / Capillary LC Column ProteCol - SGE
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| ProteCol C18 | 3 | 120/300 | 350 | 17 | 2.0-7.5 | L1 |
| ProteCol C8 | 3 | 120/300 | 350 | 10 | 2.0-7.5 | L7 |
| ProteCol C4 | 3 | 120/300 | 350 | 2.0-7.5 | L26 | |
| ProteCol SCX | 3 | 120/300 | 350 | 2.0-7.5 | L9 |
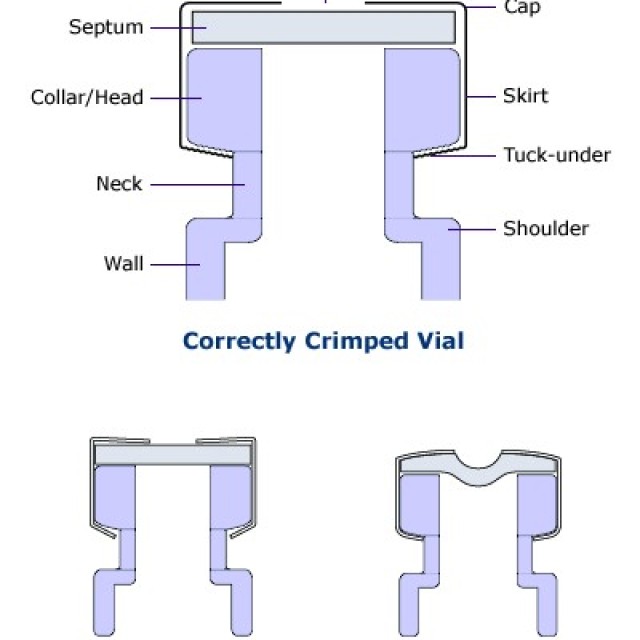
HOW TO CORRECTLY CRIMP VIALS
Crimp vials are excellent sample containers for automatic dispensers of gas and liquid chromatographs and for storing samples or calibration solutions. The technique of closing them is very important for proper tightness. Due to leakage caused by improper sealing, solvent evaporation or loss of analytes may occur.
A correctly closed vial can be recognized by the fact that its cap rotates with difficulty after closing and the septum is straight.
A vial that is closed with too much force can be recognized by the fact that its cap cannot usually be turned at all and, in addition, it has a bent septum (inwards). If the septum is punctured by the needle of the microsyringe, the septum will be heavily stressed and thus the vial's tightness will be compromised.
A vial that does not have a properly closed cap due to the low power of the crimping pliers is manifested by easy rotation of the cap and, in some cases, unfastened aluminum material around the lower edge of the vial neck.
You can set the correct force of the closing pliers.
In older types of pliers, the force is adjusted by turning the Allen key inside the jaws. Pliers also have a stop screw, which is used to set the safety distance, in order not to use too much force and thus to avoid leakage or even mechanical damage to the vial.
Introduction
Amino acids are key building blocks of life and play pivotal role in various metabolic pathways. They mostly act as intermediates often not directly involving proteins. Due to their chemical complexity and dynamic range, their reliable quantitative and qualitative analysis in biological fluids and tissues is crucial for nutritional information, compound identification and diagnostics.
For that purpose, a simple, elegant and thus far the most expeditious method for an invaluable amino acid analysis has been developed. LC/GC-MS based Metamino® kit offers comprehensive solution up to 75 metabolites including basic proteinogenic amino acids, biogenic amines and coenzymes with the possibility to a further analyte extension.
Mass chromatogram figures
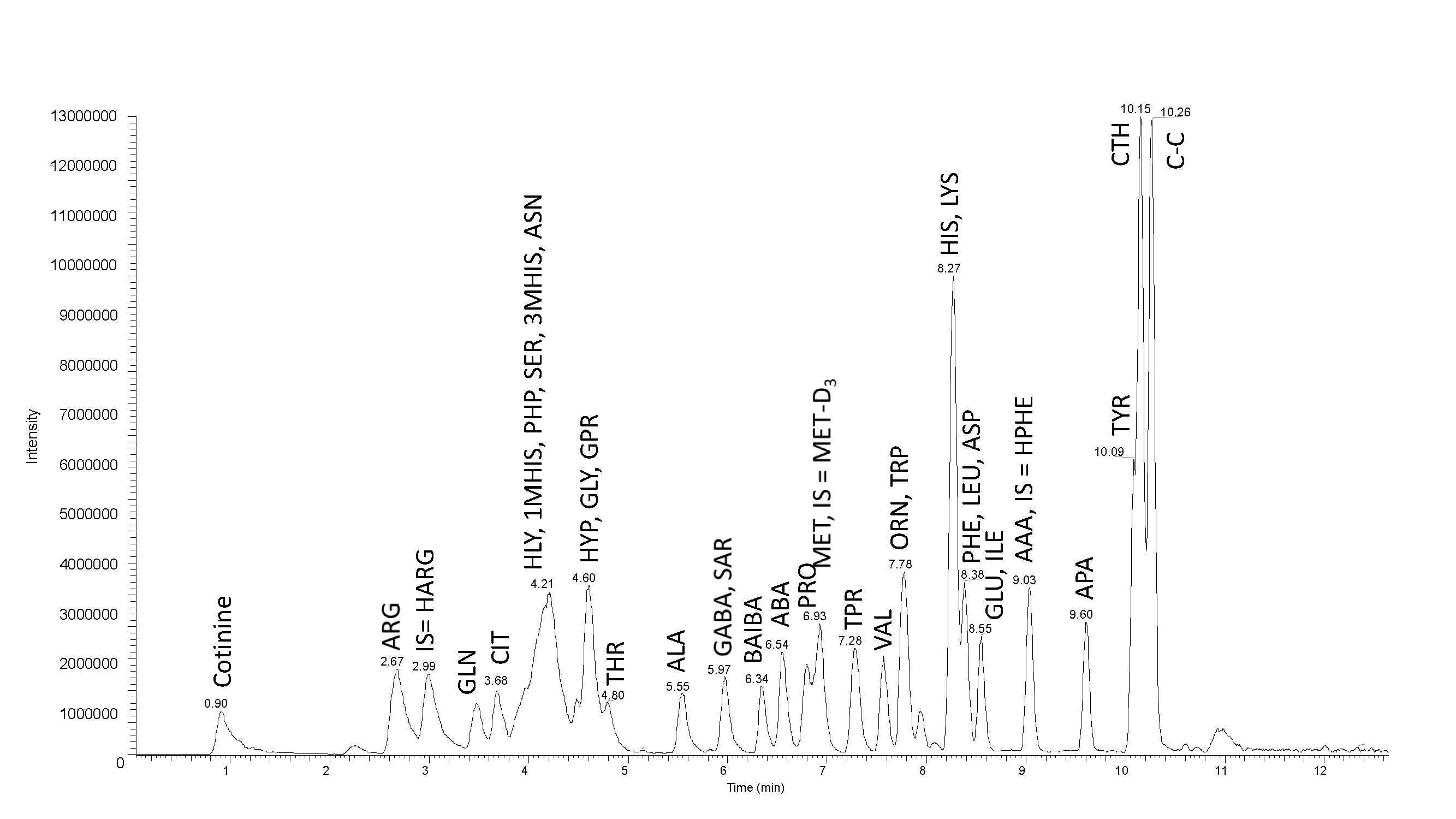
Fig. 1: Mass chromatogram of internal standards, SD1 and SD2. Separation of 5 nmoles of amino acids, using LC-MS MetAmino®
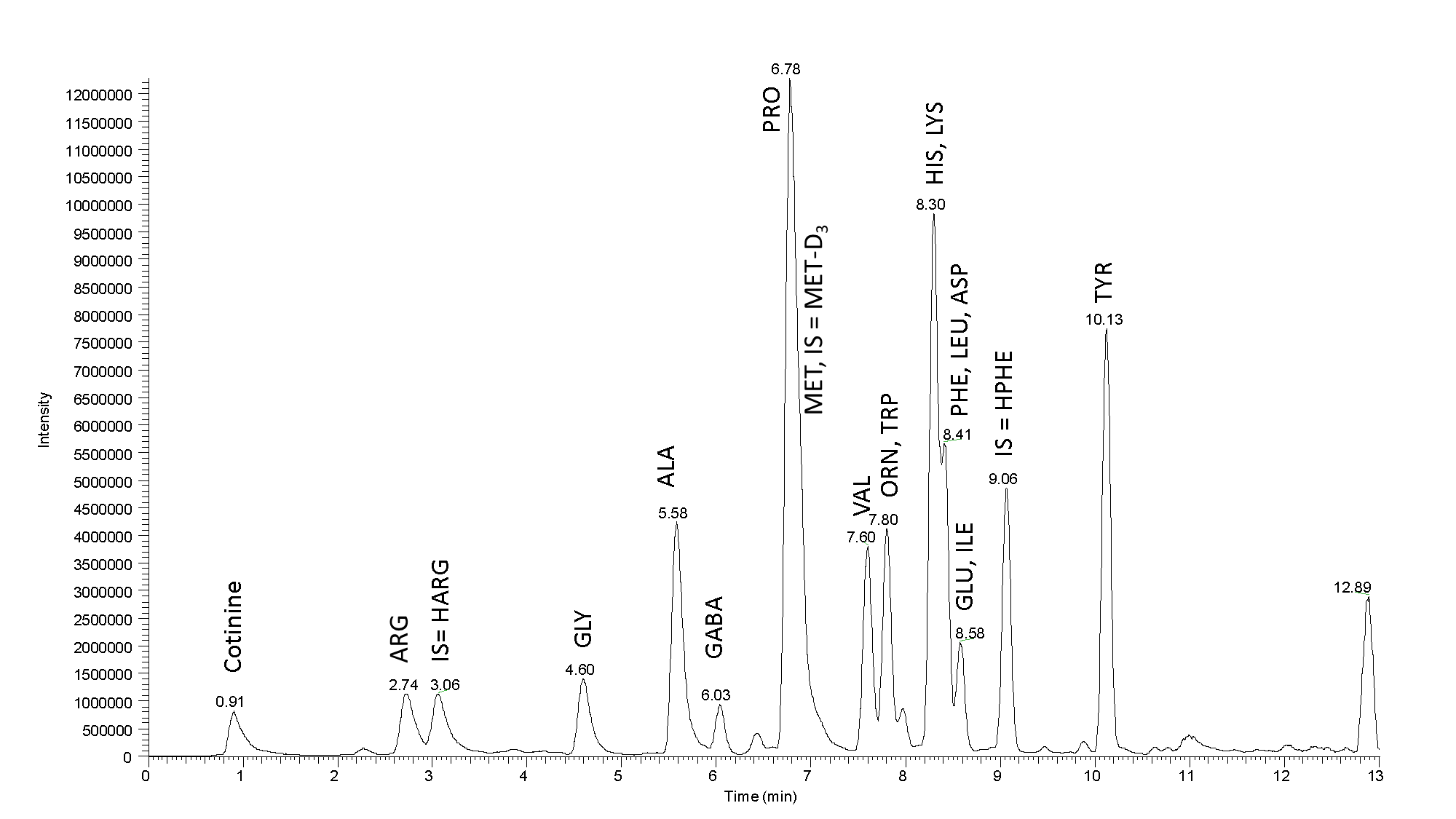
Fig. 2: Mass chromatogram a real sample: Separation of the amino acids in a sample of Budvar beer 12˚ (25 µL of the sample was the initial load). High efficiency of our column and high resolution contribute to a nice peak separation. Sample tested using LC-MS MetAmino®
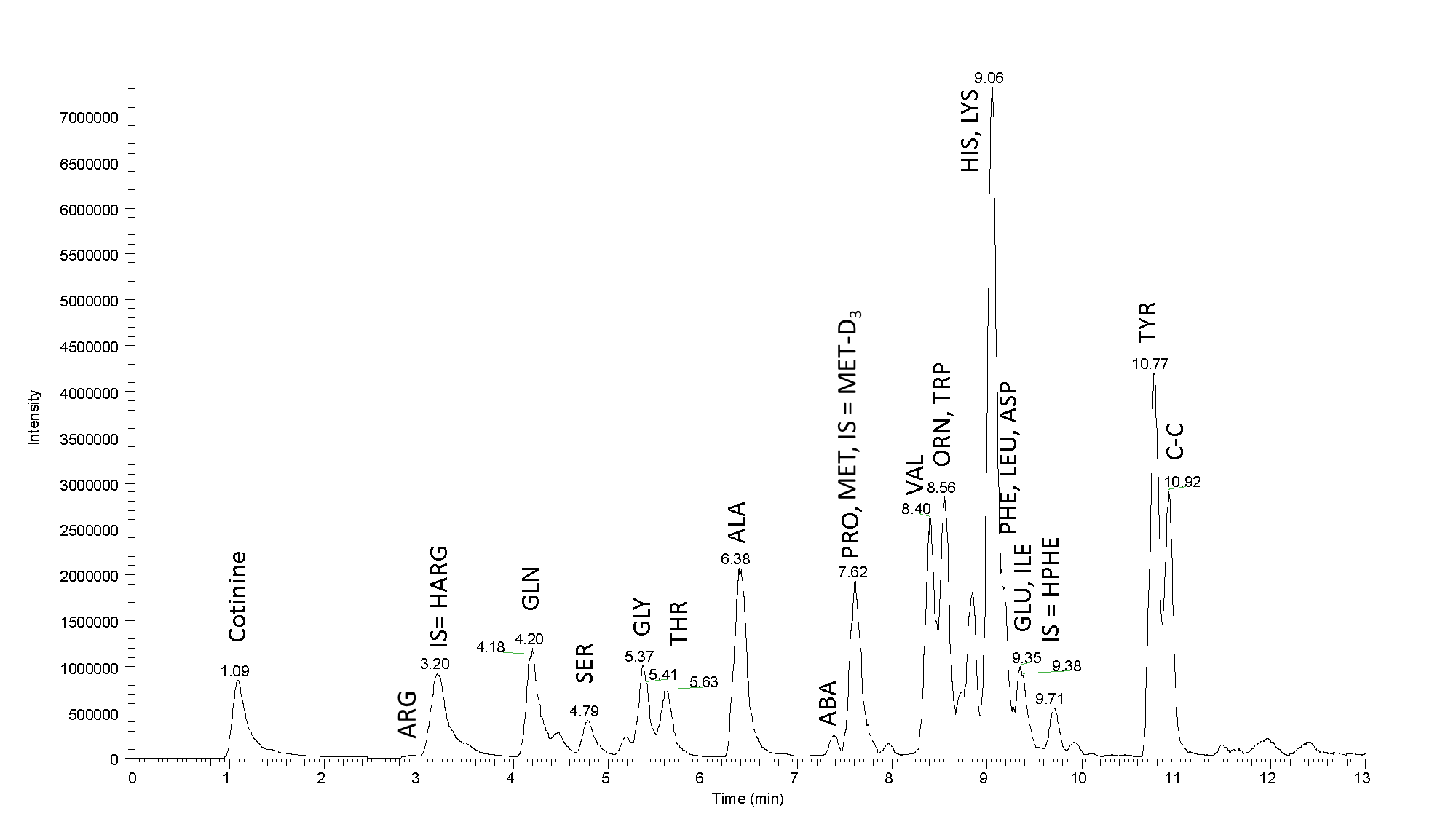
Fig. 3: Mass chromatogram of a real sample: Separation of the amino acids present in the blood serum (25 µL of precipitated serum was the initial load). High efficiency of our column and high resolution contribute to a nice peak separation. Sample tested using LC-MS MetAmino®
Sample preparation
After extracting the content out of your matrix, the sample preparation for LC-MS/GC-MS analysis can take place.
This protocol only briefly describes the sample preparation procedure. For detailed procedure see the Metamino® user manual.
LC-MS analysis goes as followed:
- Pipette 100 μL of your extracted content (serum, plasma, ...) and add 100 μL of precipitation medium. Centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Pipette 25 μL of your precipitated sample and add 10 μL of solution with internal standard to each sample preparation vial.
- Pipette 25 μL of catalytic solution into each sample preparation vial and vortex 5-10 sec.
- Pipette 10 μL of reagent solution into each sample preparation vial and vortex 5-10 sec. Let the derivatization proceed for at least 2-3 minutes.
- Activate and equilibrate the sorbent in microspin filter by adding:
- 200 μL of MSPE activation medium, then centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- 200 μL of MSPE sorbent equilibration medium, then centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Discard the flow through.
- Dilute derivatization reaction mixture with 400 μL of diluting and washing medium and vortex 5-10 sec.
- Load the diluted reaction mixture (typically 450-500 μL) to the wetted microspin filter sorbent and let it stand for 1-2 min. Centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Discard flow through.
- Wash the sorbent in the microspin filter with 200 μL of diluting and washing medium and centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Place the microspin filter into a new centrifugal vial, add 200 μL of eluting medium and centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Transfer the eluate into the autosampler vial. The sample is ready for LC-MS analysis.
GC-MS analysis goes as followed:
- Pipette 25 μL of your target sample and 10 μL of solution with internal standards into each reaction glass tube.
- Pipette 20 μL of reducing agent into each reaction glass tube and vortex briefly 5 to 10 sec. Let it stand for 1 to 2 minutes.
- Pipette 25 μL of basic medium into each reaction glass tube and add 50 μL of reagent (derivatization) solution into each reaction glass tube and vortex 5 to 10 sec.
- Pipette 50 μL of reagent (derivatization) solution medium into each reaction glass tube and vortex 5 to 10 sec.
- Pipette 25 μL of catalytic solution into each sample preparation vial and vortex 5 to 10 sec.
- Pipette 25 μL of catalytic solution into each sample preparation vial and vortex 5 to 10 sec. Let it stand for 1-2 minutes. The emulsion will gradually separate into two layers.
- Pipette 50 μL of extraction medium into each sample preparation vial and vortex 5 to 10 sec.
- Pipette 25 μL of acidic medium into each sample preparation vial and vortex 5 to 10 sec. Then centrifuge 30 to 60 sec 1,500 ×g (6,000 rpm).
- Transfer the organic (upper) layer (50-100 µL) into the autosampler vial with insert. The sample is ready for GC-MS analysis.
Kit content
The MetAmino® kit includes all reagents, liquid media and chemicals. Start-up kit content is listed in tables below:
LC-MS kit content for 100 samples:
| Item | Vial type | Volume in vial (mL) | No. of vials (100 samples) |
| Amino acid standards SD1 solution | 2 ml vial | 0.25 | 1 |
| Amino acid standards SD2 dried | 2 ml vial | - | 2 |
| Solution with internal standards | 2 ml vial | 1.1 | 1 |
| Amino acid standard diluting medium | 4 ml vial | 1.4 | 1 |
| MSPE sorbent activation medium (WES) | 40 ml vial | 22 | 1 |
| MSPE sorbent equilibration medium (EQS) | 40 ml vial | 22 | 1 |
| Catalytic Solution (CTS) | 4 ml vial | 2.2 | 1 |
| Reagent (Derivatization) Solution (RDS) | 4 ml vial | 1.1 | 1 |
| Diluting and Washing Medium (DWM) | 40 ml vial | 33 | 2 |
| Eluting Medium (ELM) | 40 ml vial | 22 | 1 |
| Precipitating Medium (PM) | 40 ml vial | 11 | 1 |
| Item | Amount (100 samples) | Note |
| MetAmino® LC Column | 1 pc | Proprietary stationary phase |
| Reagent tray for up to 80 Centrifugal Tubes | 1 pc | See Fig. 5 in Section 5.1 |
| Microspin Filters with the MetAmino® sorbent | 100 pcs | Inner tube incl. 0.22 µm membrane |
| Centrifugal Tubes (2 mL) | 400 pcs | Outer tube |
| Autosampler Vials (9 mm screw-top) | 100 pcs | Including septa and caps |
GC-MS kit content for 100 samples:
| Item | Vial type | Volume in vial (mL) | No. of vials (100 samples) |
| Amino Acid Standards SD1 Solution | 2 ml vial | 0.25 | 1 |
| Amino Acid Standards SD2 Dried | 2 ml vial | - | 2 |
| Solution with Internal Standard (IS) | 2 ml vial | 1.1 | 1 |
| Amino Acid Standard Diluting Medium (AASDM) | 4 ml vial | 1.4 | 1 |
| Reducing Agent (RA) | 4 ml vial | 2.75 | 1 |
| Basic Medium (BM) | 4 ml vial | 2.75 | 1 |
| Catalytic Solution (CTS) | 40 ml vial | 5.5 | 1 |
| Reagent (Derivatization) Solution (RDS) | 40 ml vial | 5.5 | 1 |
| Extraction Medium (EM) | 40 ml vial | 5.5 | 1 |
| Acidic Medium (AM) | 4 ml vial | 2.75 | 1 |
| Item | Amount | Note |
| MetAmino® GC Column | 1 pc | Proprietary stationary phase |
| Reaction glass tubes | 100 pcs | - |
| Reagent tray for up to 80 reaction Tubes | 1 pc | See Fig. 3 in Section 5.1 |
| Autosampler Vials (9 mm screw-top) | 100 pcs | Including septa and caps |
| Inserts for Autosampler Vials | 100 pcs | - |
Features
- In principle suitable for any matrices (urine, blood serum, tear, cerebral liquid, tissue extracts, soil extracts, etc.)
- Easy sample preparation
- Sample derivatization and analysis under 20 minutes
- Broad portfolio of analytes (75) with the possibility for a further extension
- No sample heating/ freezing needed
- NIST Library for GC/MS available
- Possible to determine substances with a low molecular weight that can be degraded in the ion source (e.g., GLY, ALA, etc.)
- Suitable for the analysis of substances that are difficult to quantify, such as polyamines
- All necessary reagents, accessories, HPLC column and clear instructions for derivatization and analysis with high accuracy and sensitivity are included
Description
Our LC-MS/GC-MS MetAmino® kits provide fast, robust, reproducible and accurate procedure for the amino acid quantification, where both sample handling and chromatographic separation are taken into account.
Using liquid chromatography (LC) coupled to mass spectroscopy (MS), the method is based on micro-solid phase extraction (MSPE) using special MetAmino® spin filter with proprietary media. MetAmino® microspin filter absorbs derivatized amino acid analytes, which are eluted through the integrated 0.22 μm membrane and afterwards injected into a MetAmino® HPLC column, undergoing a LC-MS analysis. In total, sample preparation takes around 8 minutes and sample analysis 12 minutes, thus the entire experimental time is only 20 minutes.
While using gas chromatography (GC) coupled to MS, the sample is firstly derivatized followed by liquid-liquid microextraction step (LLME). Derivatized analytes migrate into the organic layer which is then directly amenable for a quick detection on GC-MS system. In GC-MS kit, sample preparation time has been shortened to 5 minutes and sample analysis runs 12 minutes. Total experimental time is roughly 17 minutes.
This unique kit meets all emergency requirements and needs of high throughput laboratories and is designed for all end-users seeking high separation efficiency.
MetAmino FAQ
What is the type of membrane inside the spin filter?
- The membrane is made of NYLON material and its porosity is 0.22 µm. Its diameter is optimized for use with a given spin filter.
It is possible to expand the MetAmino® kit with other analytes.
- Yes, the MetAmino® kit can be further expanded. Contact us with detailed information.
May I use the kit for urine as well?
- Yes, the MetAmino® kit can be used with urine. The matrix should be without proteins. So, the sample has to be prepared accordingly (centrifugation, filtration).
Can we order the reagents separately?
- Yes, the entire set of reagents can be ordered under catalog number MAK-5857-L002.
What is the back pressure of the LC/MS column?
- The pressure is 380 bars during the beginning of the analysis, at the end it is 200 bars.
Are MRMs for amino acids given in the MetAmino® Kit were made after derivatization of the standards or before?
- The MRM transitions for AAs given in the kit manual are given as transitions of the AA derivatives and not of the native AA
Should the sample of feed be hydrolyzed before working with the MetAmino® Kit?
- Precipitation with Precipitation medium (PM) is not an essential step for successful derivatization of the sample. The kit was tested mainly for the analysis of biofluids, which often contain peptides. To avoid their precipitation during derivatization, the precipitation step was included in the sample preparation protocol.
- A total analysis of the free and peptide-bound amino acids is required. In this case, we would recommend hydrolyzing the sample and then drying the aliquot of hydrolyzed sample under a nitrogen stream or in a speedvac. We would then dissolve the dried residue in 25 µL of deionized water or 0.1M aqueous HCl (better solubility) and follow the procedure in the manual by adding 10 µL of the IS solution.
- As for the peptide hydrolysis, the additives (phenol, thiodiglycol) in the hydrolysis medium could also be derivatized (although they are probably not visible in the full scan). Therefore, we would simply recommend 6M HCl as the hydrolysis medium.
Can we order a MetAmino® GC/MS sample preparation kit for 400 samples?
- Not yet, we currently only have a MetAmino® GC/MS sample preparation kit for 100 samples. The kit can be ordered under catalog number MAK-5857-BA01.
There are 78 amino acids in table 1, some of which are not in the available mixes of standards - should we therefore rely on MRM transitions and look at the retention time or are these the standards that we should buy if we want to determine them?
- For quantification purposes the MetAmino kit contains - one bottle of SD1 standard solution included in the kit contains 33 amino acids: AAA, ABA, ALA, APA, ARG, ASP, BAIBA, CC, CIT, CTH, GABA, GLU, GLY, GPR, HIS, HLY, HYP, ILE, LEU, LYS, MET, 1MHIS, 3MHIS, ORN, PHE, PHP, PRO, SAR, SER, THR, TPR, TYR, VAL and bottle of SD2 with lyophilized mixture of 3 amino acids: ASN, GLN, TRP. However, the amino acid set can further be expanded to other compounds containing primary or secondary amino functional groups.
- The masses (m/z) reported in the manual are correct and represent M+H+ ions. The masses (m/z) and the retention times from the manual were obtained from the linear ion trap LTQ instrument however, the data from the Certificate of analysis were obtained from Q Exactive plus HRMS instrument. In both cases, the same column, flow rate, and mobile phase composition were used. The retention times (RT) are experimental values and the discrepancy especially for late eluting compounds would be caused by using different LC/MS instruments.
How can I predict how much to dilute a sample?
- It depends on the concentration of analyzed amino acids in the sample. The total amount of amino acids in the sample taken to the reaction should not exceed 1.2 μmoles. The amount of sample also depends on the LCMS instrument.
How much sample we need to take to hydrolisys with 6M HCl to use it with MetAmino® kit?
- You can take a higher amount of sample for AA hydrolysis (e.g. 50 micrograms in 300 microliters of 6M HCl) and finally take a few microliters (remove the acid in the speedvac or under the nitrogen stream) for the Metamino workflow.
In which point should the sample be diluted? After hydrolysis of the sample and before applying the kit? Or is it possible after the complete application of the kit (finished sample)?
- The reaction is designed to analyze aqueous samples, so if 25 μL of the sample gives an overloaded signal, I would recommend diluting the sample with deionized water. It is also possible to dilute the finished sample.
How much derivatizing reagent is used during derivatization process of the sample? How can I be sure that the reaction is 100%?
- The derivatization agent is sufficient to derivatize 1.2 μmol total amount of amino acids. The derivatization and the purification don´t always proceed at 100%. If you perform the derivatization of samples and calibration points in the same way, you should obtain the correct values. (Even if the effectivity of the derivatization and the micro spin purification is lower than 100%).
Overview of MSPE sorbents
Sorbents for the MSPE technique are chosen to cover the widest possible field of applications. MSPE SpeExtra C18 is a hydrophobic type of octadecyl silica gel with a special endcapping. It is suitable for a wide range of analytes, showing lower retention for polar compounds. MSPE SpeExtra C18-P is a polar modified monomeric octadecyl silica gel. It offers different types of interactions: dipole-dipole, π-π and hydrophobic. It is therefore suitable for aromatic and polar compounds. MSPE SpeExtra HLB polymer sorbent with high specific surface area and special endcapping. It has a hydrophilic and lipophilic modification ensuring universal use and a higher capacity than C18 silica gel.
| MSPE sorbent | Particle size [µm] | Specific surface area [m 2 /g] | ||
| C18 | 60 | 310 | ||
| C18-P | 60 | 310 | ||
| HLB | 30 | 850 |





 0
0
 0
0